NATO’s Trojan Horse Behind Europe’s COVID-19 Response – Part 1: Vaccinating Europe With a Military Experimental Biodefense Countermeasure
US COVID-19 RESPONSE – THE COUNTERMEASURES
• The US DoD is the Executive branch of Operation Warp Speed (OWS) and they own the "mRNA COVID-19 vaccines" until they are injected in arms.
• The National Security Council (NSC) is in charge of the COVID Policy and not HHS as they will have everyone to believe.
• The Federal Emergency Management Agency (FEMA) is in charge of Operations for the public health emergency COVID-19 response.
• HHS and their Public Health Emergency Medical Countermeasures Enterprise (PHEMCE) only act as scientific advisory group to Operation Warp Speed (OWS), they are not executive but US DoD is. Their role was only to issue recommendations and rubber stamp approvals whilst financing the R&D effort.
• COVID-19 Vaccine are in fact Security Covered Countermeasures not regulated by the FDA under Emergency Use Authorisation (EUA). The US Heath Secretary and the United States Secretary of Homeland Security must secure presidential approval to develop and manufacture security covered countermeasures (not available in the stockpile) to respond to a biological (CBRN) national security threat on the strength of a Public Health Emergency and when an Emergency Use Authorisation (EUA) has been authorised by the FDA Commissioner.
• The Biomedical Advanced Research and Development Authority (BARDA) under the dome of HHS, is in charge of financing the development and manufacturing of these countermeasures. They also seems to have taken on the role of a regulator which they are not.
• Advanced Technology International (ATI) micro-manages Big Pharma OTA contracts for the US DoD and for BARDA. They also have an oversight over the contracts allocated to pharma companies members of the Medical Technology Enterprise Consortium (MTEC) and the Medical CBRN Defence Consortium (MCDC).
• FDA mandate does not provide them with the legal authority to regulate Security Covered Countermeasures under EUA. The law is clear… Development & Manufacturing of countermeasures under EUA cannot be considered to constitute a clinical investigation, meaning none of the clinical trials including subjects, investigators and trials outcomes exist within the context of the US code (as Pfizer demonstrated during the Jackson Brook law suit).
• FDA reviewed the Pfizer clinical trials data for the Pfizer BNT162b2 mRNA shot before trying to bury it for 70+ years as the “Cumulative Analysis of Post-authorization Adverse Event Reports” were confirming an inconvenient 1,223 fatal cases and 42,000 adverse reactions as part of Pfizer clinical trial outcome” (source section 5.3.6)
• Pfizer and big pharma were contracted by US DoD and the Biomedical Advanced Research and Development Authority (BARDA) using Other Transaction Authorities (OTA) a form of contract which by nature drastically reduces vital oversight opportunities and protect IP meaning the US taxpayers never get to know what’s actually in the jab and same goes for US Government agencies which too will find it very hard if not impossible to get clarity or access.
• OTA contract confirms that Pfizer was contracted for a demonstration (demo) of a prototype mRNA vaccine in view of large scale manufacturing. This does not make their product a pharmaceutical product as demo and prototype are not subjected to strict manufacturing regulatory measures and protocols but only require “reasonable efforts” on the manufacturers part . Clinical trials data can be incomplete and it is not required for the vaccine companies to prove safety or efficacy when SOW is a demo and a prototype.
• FDA / CDC and mass media were deceitful and misleading by promoting efficacy and safety of these injections as we now know immunity studies were never conducted prior to big pharma receiving their initial marketing approval. (this was confirmed by Janine Small from Pfizer). People were told sometimes even forced to get the jab to keep their job and to allegedly protect others when FDA and CDC had no idea whatsoever if the jab could stop infection and transmission.
• We know NIH have been sponsoring Game of Function for a very long time, in and out of the United States and even when Obama’s moratorium forbidding GOF research was in place
• SARS CoV alleged isolated virus ( for the virus therory believers) was patented by the CDC hence it cannot be fully natural and if it was fully natural, it was illegally patented. US7220852B1 - US7776521B1
• COVID-19 so called vaccines are not pharmaceutical products as they are not strictly regulated, they are not prophylactic, they do not stop infection nor transmission and they do harm people. This experimental products and delivery systems were not developed in good faith since we can establish from available early data that Big Pharma and FDA had prior knowledge of their dangerousness which establish intent to harm. The above information pertaining to these injectables qualifies and characterises this COVID-19 injections as a “Military grade Security Covered Countermeasure” and by definition as a “Bioweapon” or “Synthetic Bioweapon”
What has been reported so far about the Covid-19 injections?
- mRNA ‘vaccine’ material does not stay at the injection site, but instead can travel throughout the body and accumulate in various organs,
- If the Pharma industry’s claims about the mRNA technology are correct, then the COVID vaccines may induce long-lasting expression of the “SARS-CoV-2 spike protein” in many organs,
- mRNA vaccine-induced expression of the toxic ‘spike protein’ could induce autoimmune-like inflammation,
- and vaccine-induced inflammation triggered by injection contents, including Lipid Nanoparticles (LNP), cause grave organ damage, especially in vessels, sometimes with deadly outcomes.
Vascular and organ damage are clearly induced by mRNA vaccines, and here is additional scientific proof of causality. Why was studies such as immunohistochemistry never conducted nor requested by government regulators to detect either the widespread expression of ‘spike protein’ or reactions to LNPs in animal trials during preclinical development? This question burdens the minds of people like Prof. Arne Burkhardt, a very experienced pathologist from Reutlingen, Germany, who heavily contributed to this August 2022 article published on the Doctors for COVID Ethics website. With the help of his colleague Prof. Walter Lang, Prof. Burkhardt has studied numerous cases of death which occurred within days to several months after vaccination. In each of these cases, the cause of death had been certified as “natural” or “unknown.” Burkhardt became involved only because the bereaved families doubted these verdicts and sought a second opinion.
It is remarkable, therefore, that Burkhardt found not just a few, but the majority of these deaths to be due to vaccination. Clearly, something has gone terribly wrong here, and so it forces us to reflect on the central question:
“If these mRNA Covid-19 vaccines were not safe, not efficacious, not prophylactic, and were harming people (which is what a Bioweapon does) why would regulators such as FDA, MHRA, EMA, Health Canada, etc….would grant vaccine companies marketing approval?”
Burkhardt Group Conclusions can be found here.
With this basic knowledge in hand, it has become clear that the people of Europe need to start questioning the true nature of the COVID-19 pandemic and also investigate the biodefense programs that have flourished over the years in countries like US, Georgia, Germany, Ukraine, and China just to name just a few. The need to increase the debate regarding the safety and efficacy of these Biodefense COVID-19 ‘countermeasures’ (pandemic policy measures and ‘vaccines’) is being felt more than ever, and so part of our recent discussions with various parties was to establish the level of awareness regarding the European Commission and its regulator, the European Medicines Agency (EMA) and the control they actually had over these US military bio-defense countermeasures which were granted “Conditional Marketing Approval” (CMA) characterised as “mRNA COVID-19 vaccines” by these two institutions.
Is there a transnational Biodense Mafia?
While the Soviet Union stumbled and lesser nations mixed radical ideology with a commitment to counterbalance the West’s conventional advantage, the revolution in biotechnology accelerated.
The human genome project, genetic engineering, synthetic biology, and recombinant technologies were carrying the promise of a better life, but at the same time reminded us all that, historically, beneficial technological advancements are sometimes exploited by unscrupulous people, often driven by profits ( like big pharma). Non-biological terrorist incidents close to home have raised our awareness and concern that a new generation of corporate biological terrorists might use bacteria, viruses, toxins, or even designer agents against populations to justify the deployment of expensive covered countermeasures known to us as vaccines.
Throughout the cold war, proliferators had independently selected nearly the same list of weapons agents for tactical use on the battlefield or strategic use to be delivered by intercontinental ballistic missiles. It was a relatively small group (10-20) of agents drawn from hundreds available in nature that had physical and biological characteristics such as pathogenicity, toxicity, ease
of production and stability qualifying them for aerosol dissemination.
The following quote comes from a book written by Dr. David R. Franz titled Medical Countermeasures to Biological Warfare Agents. This book is part of NATO Sciences Book series (ASDT, volume 34). Dr. Franz is a Doctor of Veterinary Medicine and a former Commander United States Army Medical Research Institute for Infectious Diseases (USAMRIID) and this is what he wrote:
“To produce a true mass casualty attack the terrorist will likely have to use agents from that threat list developed and tested for biological warfare application. However, because a small non-lethal attack, or even a biological hoax, is adequate to cause panic and lead to coveted national television coverage, the modern bioterrorist probably has a much wider spectrum of agents from which to choose. However, the terrorist spectrum, while much broader, is not necessarily more lethal.”
Many have profiled Robert Kadlec, Christian Hassell, or bio-weapons researcher Steven Hatfill even Dick Spertzel whose government careers go back to the 2001 anthrax attacks and the FBI’s Amerithrax investigation. However, three individuals seem to have operated in a league of their own. The relationship between the Wuhan Institute of Virology (WIV) and the American Biodefence establishment was advanced by EcoHealth Alliance policy advisor, David R. Franz (quoted above) who also was a former commander at US bioweapons lab at Fort Detrick and another interesting character, Dr. Thomas Geisbert, a biodefence/biowarfare expert at the Galveston National Laboratory. The third individual would have to be the legendary DARPA man Dr. Michael Callahan.
Could these people, who seem to share the same values, be at the very center of the most elaborate hoax of all time, the COVID-19 plandemic? I am pretty confident about my answer to this question, but time will tell…
Although the concept and stories surrounding the use of biological weapons are as old as King Herod, modern technology brings many new possibilities, especially with the field of synthetic biology, recent advances in gene editing technology, and so much more – it seems that the sky is no longer the limit. A US Intelligence Community report published in 2016 provided a Worldwide Threat Assessment. It was written by James Clapper, former director of National Intelligence, and technologies such as “Genome Editing” were listed in the “weapons of mass destruction” and proliferation section:
“Research in genome editing conducted by countries with different regulatory or ethical standards than those of Western countries probably increases the risk of the creation of potentially harmful biological agents or products. Given the broad distribution, low cost, and accelerated pace of development of this dual-use technology, its deliberate or unintentional misuse might lead to far-reaching economic and national security implications”.
Following World War II, during the Cold War, the United States biological warfare research was stepped-up and was turned into a substantial, military-driven industry of research and development. However, the manufacturing program was wrapped up in secrecy. Hence, it’s hard to know which biowarfare tools have real-world applications, and which ones are merely in the protracted R&D stage.
In 1998, as a response to the perceived bio-threat posed by the likes of Iraq, the US DOD embarked on a project to inoculate over a million US troopers against Anthrax. Many refused the vaccine and were sanctioned, whilst others suffered horrible side effects. The BioThrax vaccine’s sole producer was once a business enterprise known as BioPort funded by German-born Fuad El-Hibri and Admiral William J. Crowe Jr., a former chairman of the Joint Chiefs of Staff and President Bill Clinton’s Ambassador to the United Kingdom.
Fuad El-Hibri was one of Robert Kadlec’s partners in crime. Kaldec was a US Air Force physician who also served as the Assistant Secretary of Health and Human Services (2017-2021), and was no less than one of the masterminds behind shaping the Pentagon’s biodefense ambitions. Kadlec is part of a tight-knit group of “bioterror alarmists” and business-minded operatives embedded in government agencies and also the private bio-military contractor sector. Under his aegis, the program became a much more opaque department, largely accountable unto itself. Corruption tends to breed under such conditions. Kadlec was the mastermind behind the rise of the new “Biotech-Industrial Complex” that captured the attention of many top officials across the federal government, as well as federal agencies, including the HHS, BARDA, and the U.S. Department of Agriculture. His coup de grace was to manage it all through a cabinet-level steering committee overseeing all federal biodefense activities and… funding budgets.
Vaccines: Follow the Money
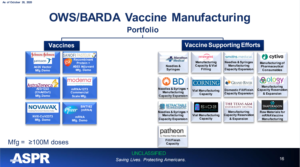
BioPort eventually changed its name to Emergent BioSolutions and was, much later, contracted to produce the new COVID-19 vaccines as confirmed by this Vaccines and Related Biological Products Advisory Committee October 22, 2020, Meeting ASPR Presentation.
If you are interested in more details, visit Whitney Webb and Raul Diego’s investigative series, “Engineering Contagion” which will certainly quench your thirst, and likely convince you that indeed all roads lead back to the Dark Winter initiative.
I also invite you to read Spartacus’ “Biodefense Mafia” article, and also watch his latest video here:
Not everyone enjoys spending hours identifying laws, rules, and protocols that apply to governments, regulators, and Big Pharma, but if we are to get to the bottom of this unprecedented attack on the global population, we must at least understand the foundation behind this man-made “extermination program” concealed under the guise of a ‘global pandemic.’
The root of these biodefense programs can be traced back to 1969 when the US sat up law 50 U.S. Code Chapter 32 to establish chemical and biological warfare programs carefully crafted to deceive the public by pretending such programs were Protective, Prophylactic, and Defensive in nature. Sadly, when it comes to the experimental Covid-19 ‘vaccine’, this couldn’t be further from the truth.
The legal path borrowed by these Biodefense programs and their architects is complex, and to comprehend how they came about and the necessary legal framework to bring them to life – is in itself a brainstorming exercise. But thankfully, we have been gifted with the work of professional paralegal, Katherine Watt, who has skillfully managed to break it down for all of us. You can find her work at the Bailiwick News Substack. Here is also a link to her “Kill Box” presentation.
On January 24, 2023, Katherine Watt was an attendee at a press conference that discussed the ongoing emergency use roll-out of bioweapons being marketed as Covid vaccines, and here is an extract from her presentation (source):
It is rather scary to see how many people within this criminal public partnership enterprise were, and still are, more than happy (not to say excited) about enslaving the rest of us. However, we are still here alive and kicking and willing to press on to discover the true nature of the beast and establish how much was done to bring so many into a state of catatonic compliance.
I propose we now take the time to review the process that was put in place in the European Union to mask the obvious intent behind this Plandemic and see what it actually took for these Synthetic Biology Enabled Weapons (SBEW) to reach the arms of the EU citizenry. Further down in this article, we will address the topic of synthetic biology and how it enables bioweapons development.
The procedure used by the EU to approve COVID-19 vaccines aka ‘medical countermeasures’:
A medical product for human use may be authorized in the EU under Directive 2001/83/EC19 and Regulation 726/200420 either by the EU Commission following favorable advice from the EMA through a centralized procedure or by competent national authorities under national procedures. For human medications having a newly created active ingredient, the centralized method is required. Most generic medications are evaluated and approved at the national level, but the majority of original, innovative medicines, including vaccines, are subject to the centralized authorisation system.
A single marketing authorisation application, detailing the safety, efficacy, and quality of the vaccine must be submitted to the European Medicines Agency (EMA) under the centralised process – by each vaccine developer who wants to commercialise a vaccine in the EU. Three specialised committees are involved in the EMA’s evaluation process:
1. The Pharmacovigilance Risk Assessment Committee assesses the safety of the vaccine.
2. The Biologics Working Party verifies its quality
3. The EMA’s Committee on Human Medicines (CHMP) – which includes members from all Member states – issues the final recommendation to the EU Commission if the evidence shows that the benefits of vaccination are greater than any risks from the vaccine.
The Process:
Within 210 days of receiving a valid application, the CHMP renders its opinion. Following a favorable CHMP recommendation, the Commission examines the validity of each component supporting the marketing authorization. These cover the following: scientific rationale, product details, information for healthcare experts, labeling, and vaccination usage guidelines. Through the comitology procedure, the Commission contacts EU Member states that are in charge of the domestic marketing and use of the vaccine before authorizing it.
The Conditional Marketing Authorization (CMA) is enforceable once issued in all Member states. The EMA and Member state keep an eye on the vaccine’s safety once it has been authorized for use, and take action if new information suggests that it is no longer as safe and effective as it once was.
Was the development and approval process for Covid-19 vaccines sped up because of the urgency of the situation and the need to find a solution to stop the spread of Covid-19 or was the process actually hastened to use the panic effect and the distraction manufactured by our mass media and health authorities to accelerate the deployment of these US Biodefense Countermeasures on European soil?
The normal process for developing vaccines was condensed, and various phases of their development were combined, only making it feasible to accelerate the production process. The EMA started evaluating the vaccine before the regular laboratory checks had been finished, and clinical trials began before the in vitro and in vivo studies were finished. Some producers began making their vaccines prior to receiving EU marketing authorization. As a result, vaccinations were made accessible for use and distributed throughout the EU before the usual process for their development was finished.
Regarding the approval process, a number of agreements made it possible for the EU Commission to make a decision more quickly, resulting in the approval of the first vaccination, such as the Comirnaty Countermeasure created by BioNTech and Pfizer only nine months after the pandemic was proclaimed.
First, the Commission and Member states decided to activate the conditional marketing approval (CMA) procedure in order to hasten the approval of Covid-19 vaccinations in accordance with Article 14 (7) of Regulation 726/2004 and the conditions of Regulation 507/2006. This is in accordance with the provisions of Article 21 of Regulation 1234/2008 which allows the Commission to accept a change to the conditions of a marketing authorization for a human influenza vaccine in cases where some non-clinical or clinical data are missing on an exceptional and temporary basis during a pandemic. Under the CMA framework, marketing authorization is given on the basis of less extensive clinical data than is typically necessary where the advantages of the medicine’s prompt availability outweigh the risks associated with the need for further evidence, which naturally puts everyone taking the procedure at risk.
To expedite clearance during public health emergencies, the CMA is employed as a fast-track authorisation mechanism.
On December 1, 2020, both Pfizer and BioNTech submitted an application for Conditional Marketing Authorization (CMA) for COVID-19 Vaccine to the EMA, meanwhile, the European Commission was proposing rules which could give the EU the power to declare a “Public Health Emergency” which was required to justify the fast-tracking of CMA approvals.
A CMA may be obtained in accordance with Article 4 of Regulation 507/2006 if the EMA’s Committee on Human Medicines determines that all of the following conditions have been satisfied despite the lack of full clinical evidence pertaining to the safety and efficacy of the medicinal product:
(a) The medical items have a favorable risk-benefit ratio
(b) The likelihood that the applicant will be able to produce thorough clinical data is favorable;
(c) The drug will enable meeting unmet medical demands; and
(d) The quick market availability of the relevant pharmaceutical product is advantageous to public health and outweighs the risk associated with the need for more information.
In the “whereas” section (4) it reads:
Where Conditional Marketing Authorisation (CMA) are granted, they should be restricted to situations where only the clinical part of the application dossier is less complete than normal. Incomplete pre-clinical or pharmaceutical data should be accepted only in the case of a product to be used in emergency situations, in response to public health threats.
This explains why in November 2020 the European Commission was desperate to establish rules allowing the EU to declare a public health emergency in future outbreaks independently from the World Health Organisation (WHO). For that Ursula Van der Leyen the European Commission President had already few cards up her sleeves like the good magician she is; making her SMS conversations with Pfizer CEO Albert Bourla disappear was just a warm-up act, “The Pledge” before “The Turn”, and finally “The Prestige” – with the announcement in September 2020 of the launch of a brand new EU agency called the “Health Emergency Preparedness Authority” (HERA), a key pillar of their European Health Union project which will mirror the US Biomedical Advanced Research and Development Authority (BARDA). This is a de facto merger of the transatlantic biodefense space. Indeed, the New York Times sued the EU over Ursula Von der Leyen’s Pfizer missing text messages, but what we are truly interested in is the full content of the Advanced Purchase Agreement (APA) between vaccines companies and the European Commission, and the thirty pages of these contracts which still remain redacted.
Clearly, this is a cover-up at the highest levels – at minimum three-way collusion between the transnational Pharma-Biomedical Industrial Complex, the United States, and EU governments. At worst, we are witnessing the birth of a global Private-Public Partnership fascist cartel.
But there’s more…
On 17 February 2021, the European Commission launched its new “bio-defense preparedness” plan designed to bolster Europe’s preparedness against the looming threat of new coronavirus strains, which includes moves to fast-track future ‘variant’ vaccines – a concept aggressively promoted by Bill Gates and other key players.
The plan, known as the “HERA incubator”, was unveiled by the Commission, explaining how HERA will work with researchers, biotech companies, manufacturers, and public authorities in the EU and globally to detect and prevent the spread of new variants” read this EURACTIV article.
European Commission then launches the HERA Incubator on Wednesday 17 February 2021.
Online public consultations on the Health Emergency Preparedness and Response Authority (HERA) were conducted starting on the 31st of March 2020, just two months prior to WHO Director-General Dr. Tedros Ghebreyesus declaring the ‘novel’ SARS COV2 a “public health emergency of international concern” (PHEIC).
Will EU watchdog organisations will investigate this public consultation on HERA and establish when this plan was actually put together? Was it before or after Dr. Tedro’s announcement?
November 2020 was also the month Guido Rasi, head of EMA, suddenly stepped down, handing the running of EMA to Irish national Emer Cooke on a strange procedural formality. Coincidence?
In the US, it was the responsibility of HHS Health Secretary Alex Azar (a career Big Pharma insider and executive) to declare a “Public Health Emergency” in order to trigger an FDA and BARDA “Emergency Use Authorisation” (EUA), an effective carte blanche for any and all products and policies to go into motion. However, in Europe, the WHO’s declaration was enough to allow the European Medicines Agency (EMA) to regulate the US DoD Biodefense ‘countermeasures’ and their rapid deployment (with no oversight) on European soil under the rules and guidelines applicable under “public health emergency”, which had a consequence of exposing all EU citizens to an obviously unproven, unsafe, inefficacious and poorly manufactured product – fraudulently sold to governments as ‘safe and effective’ pharmaceutical and medical products, disingenuously labeled as “vaccines” – as they do not provide immunity or inoculation from any pathogen, and merely “reduce mild symptoms of illness”, then they cannot be labeled as vaccines, but rather as pharmaceutical treatments. But under the fog of biowarfare, this Department of Defense-led operation cares not about labels, because everything falls under the unassailable shield of military “medical countermeasures.” And herein lies the problem.
SEE ALSO: Are COVID-19 ‘Vaccines’ a Military Biodefense Response Gone Terribly Wrong?
21 December 2020, the European Commission granted a CMA for the COVID-19 ‘vaccine’ developed by BioNTech and Pfizer, making it the first COVID-19 vaccine authorised in the EU. This authorisation followed a favourable scientific recommendation based on an assessment of the safety, effectiveness, and quality of the so-called vaccine by the European Medicines Agency (EMA) and was subsequently endorsed by the EU Member States. No questions asked. After all, it was supposed to be a “public health emergency.”
It is accepted that a novel vaccine candidate needs to be evaluated for “safety, immunogenicity, and protective efficacy” in humans before it is licensed for use and unleashed on the population, but here we saw the European Commission (not the EMA) granting a Conditional Marketing Authorisation (CMA) for the Comirnaty mRNA COVID 19 vaccine developed by BioNTech/Pfizer (along with the U.S. DoD) without thoroughly testing for immunogenicity, which is essential to establish the ability of a molecule or substance contained in the shot to provoke a so-called “immune response” (or toxic reaction, depending on how you view the science), but also the strength and magnitude of an immune response which might affect the safety profile of these so-called ‘vaccines’, as well as their ability to stop transmission (especially when the initial data was exposing the lack of integrity of these experimental mRNA injections).
The Regional President of Pfizer Vaccines in the International Developed Markets (IDM) Janine Small admitted to the EU Parliament in Oct 2022, that prior to Pfizer being granted their first CMA no efficacy and immunogenicity tests were conducted. To use her own words, “We had to work at the speed of science”, whatever that means. In other words, Pfizer had no clue if their injection would stop people from getting infected, or from transmitting the so-called virus. Worst of all, they were playing a biblical game of Russian roulette with the European population.
Furthermore, Pfizer had determined the pharmacokinetics of the two novel LNP excipients ALC-0315 (amino lipid) and ALC-0159 (PEG-lipid) in plasma and liver, as well as their elimination and metabolism in rats, but not in humans. How and where these nano lipids were distributed in your body was unknown – representing another reckless and totally unaddressed safety issue.
As far as clinical pharmacology is concerned, Pfizer lodged a Biologic Licence Application (BLA) with the FDA in Washington DC on May 18, 2021, and section 5 of the BLA is without appeal.
COMIRNATY induced a “humoral immune response”, but for some reason, Pfizer has no idea how. (source)
The EMA’s ‘rolling review’ process significantly shortened the normal assessment time of these experimental injections.
During the review, a Covid-19 EMA pandemic task force (Covid-Etf) was established to support EMA and its scientific committees. It brought together experts from across the European medicines regulatory network to advise on the development, authorisation, and safety monitoring of vaccines. How could all these experts not see something wasn’t right with the data?
The EU Commission also made sure that the procedure for obtaining the marketing authorization for vaccines moved as quickly as possible (hurriedly) by cutting the time required for Member States’ comitology consultations short and allowing translations of all pertinent documents into the full range of languages only after the authorisation had been granted. As for the Covid-19 vaccines, the Commission was able to move forward with a marketing authorization within just 3 days after receiving a ‘favorable’ recommendation from EMA. The average standard process for the authorisation of medicinal products takes at least 67 days, including 22 days for the consultation of Member States, so what happened here? Why were normal safety checks and balances discarded?
That’s right: just 3 days… that’s how much time the EC invested to ensure that European citizens’ safety was addressed.
In line with the EU safety monitoring plan for COVID-19 vaccines, Comirnaty was supposed to be closely monitored and subjected to several activities that apply specifically to COVID-19 vaccines. But did this really happen? Although large numbers of people and rats had received Pfizer COVID-19 vaccines in clinical trials, certain side effects had to be expected after millions of people were injected with the experimental product.
The legal basis is Article 14-a of Regulation (EC) No 726/2004. The provisions for granting a “conditional marketing authorisation” for Comirnaty are further elaborated in Regulation (EC) No 507/2006.
As you can read on the EMA website it is up to EMA and their Committee on Human Medicines (CHMP) to grant Conditional Marketing Approval (CMA) so why was the CMA granted by the European Commission – and not by the EMA? Was this done by executive fiat, in order to bypass any potential oversight of the fast-tracked experimental product?
We have to keep reminding ourselves that there is a very serious risk associated with the use of new vaccines (countermeasures). The creation and approval of Covid-19 countermeasures are contingent upon the production of scientific evidence demonstrating that the vaccine’s risk-benefit ratio is still favorable and that the benefit to public health of its immediate availability overwhelmingly outweighs the risks associated with the injury and death of innocent civilians. Clearly, there was a need for more safety and efficacy data, but these concerns were silenced.
The risk-benefit balance should have been put under the microscope a long time ago, as today the data seems to be contradicting the very questionable assessment made by EMA/CHMP. The debate regarding people’s injuries and deaths following the Covid injections is still very much censored today (as part of 5th Generation Warfare?), and very few governments seem interested in talking about it, and same goes for the deafening silence from so-called ‘regulators’ like the FDA, CDC, and of course the EMA/CHMP.
A few brave Members of the European Commission have been fighting for some transparency, but as things currently stand as of March 2023, we Europeans are yet to be been given access to the Advanced Purchase Agreement (APA) which Ursula van Der Leyen has discretely negotiated with Albert Bourla the CEO of Pfizer. All SMS and communications pertaining to this negotiation still remained hidden from the public, but also from the EC auditors. All information pertaining to this matter much be made public immediately. Failure to do so only confirms that a serious cover-up is underway.
Corruption is Crippling the EU
Europe’s lack of transparency will be its demise with cases of corruption surfacing one after the other. Recently, the arrest of vice president of the European Parliament Greek socialist MEP Eva Kaili (and her gang), not to mention the corruption scandal which unfolded in May 4, 2021, as €4M suddenly appeared on Stella Kyriakidesthe, the EU Health Commissioner’s bank account – which she holds jointly with her husband Kyriakos Kyriakidesthe. In her declaration of financial interests, Commissioner Kyriakides had listed a number of ‘management positions’ for her husband Kyriakos Kyriakides, however, the company MARALO LIMITED did not appear, even though Kyriakos Kyriakides is listed as its managing director. Large loans had been taken out for the company, but these loans, which were granted to the company by the Cyprus Cooperative Bank, were recently reported by the Audit Office of the Republic of Cyprus and couldn’t be justified.
Corruption is clearly at the heart of the EU, and scandals such as the ones mentioned above are just the tip of the iceberg. You can be sure that many emails and SMS messages are being deleted as we speak.
In early 2020, it was made clear by the President of the European Commission Ursula van der Leyen, that these secretive Advanced Purchase Agreements with Big Pharma had a specific condition attached to them: mRNA COVID-19 vaccines would be manufactured in Europe. Her statement was slightly irrelevant, not to say misleading, since she knew these countermeasures were developed and processed in the United States, a fact which we are going to demonstrate in a moment.
The large-scale manufacturing of (unprecedented) mRNA vaccines in such a short amount of time implies that raw materials (200+ ingredients) and manufacturing capacities, would have had to be already available and secured, on standby if you will, and not ‘on demand’ as claimed by politicians.
Furthermore, anyone working in the drug manufacturing industry will tell you how tricky it is to streamline production plants for injectables under cGMP (current good manufacturing practices) and CMC (Chemistry, Manufacturing & Controls) regulations to ensure product safety and consistency between batches. This takes months, if not years of preparations, and constant testing.
Looking into Moderna and Pfizer SEC fillings, we can appreciate that these vaccine companies were mainly contracting CMO which are Contract Manufacturing Organisations in this case for “Fill & Finish contracts” in Europe. The vaccine development effort was conducted mainly in North America.
BioNTech started their own manufacturing process for their COVID-19 vaccines in February 2021 at the new Marburg facility in Germany with the execution of the first step: the production of mRNA, which is the alleged active pharmaceutical ingredient, which BioNTech announced in a statement.
This new BioNTech site was to become one of the largest mRNA manufacturing sites in Europe with an annual production capacity of up to 750 million doses of our COVID-19 vaccine, once fully operational. BioNTech’s plan was to produce up to 250 million doses of BNT162b2 in the first half of 2021. The first vaccines manufactured at the Marburg site were scheduled for distribution in early April 2021.
According to BioNTech, after purification, lipids are to be added to form lipid nanoparticles (LNPs) — the delivery vehicle for the drug. The purified and concentrated product would then be transported to a “production partner” site for completion under sterile conditions (fill and finish).
The main EU plant for the production of the vaccine is Pfizer’s factory in Puurs, Belgium.
We believe it is fair to assume that the several hundred million COVID-19 vaccines which were deployed on European soil between December 2020 and April 2021 – were not manufactured in Europe, but in the United States.
Page 6 of Pfizer’s July 2020 OTA’s contract with the US Department of Defense indicates in Section C of the document. Chemistry Manufacturing Controls (CMC) that to ensure delivery of the doses (countermeasures) Pfizer had to continue with BioNTech manufacturing initial clinical trial material for the EU and US 1/2/3 studies through mRNA production site in Germany and the EU using Pfizer Belgium based ‘fill and finish’ site in Puurs. (demonstration for a prototype as per OTA SOW).
Therefore, the manufacturing of the “Initial Clinical Trial Material” was conducted under the understanding that this was not related to the large-scale manufacturing demonstration they were contracted to do by the US Department of Defense, and therefore out-of-scope for the US DoD-ordered prototype project and was, therefore, to be conducted at the expense of Pfizer.
BioNTech and their chosen Contract Manufacturing Organisation (CMO) transferred the complete knowledge of the initial trial material manufacturing to Pfizer in order to establish the entire process at the Pfizer site in the US where the development and large-scale manufacturing for these security-covered countermeasures was to take place. This again proves our point that the large-scale manufacturing of these (military-run) ‘countermeasures’ took place on US soil, and not in Europe.
Then the question is:
“…how the European regulator exercised its authority over Security Covered Countermeasures developed and manufactured in the US under FDA emergency use authorization (EUA) and in partnership with the US Department of Defense?”
In 2021, both companies worked jointly to commercialize the vaccine worldwide, excluding China, which was already covered by BioNTech’s collaboration with the Chinese CCP company Fosun Pharma. In their OTA contract, the success of the prototype is characterised as followed:
“Success of the prototype project in this OTA is defined as the manufacture of 100M doses of Pfizer and BioNTech’s mRNA-based COVID-19 vaccine and, upon FDA-approval or authorization”
Clearly, there is no mention of EMA approval here.
Extract from Pfizer July 2020 OTA with the US Department of Defense
The fact of the matter is that these injections were developed and deployed by vaccine manufacturers under the leadership of the US DOD with regulators such as the FDA and EMA very much aware that efficacy couldn’t be established – since no immunisation studies were ever conducted (as we have demonstrated earlier).
The COVID-19 response and its countermeasures were facilitated by HHS and EU declaration of Public Health Emergency and subsequent emergency use authorizations (EUA) that had the effect of removing all of the strict regulatory measures normally imposed on Big Pharma designed to prevent exposing civilians to unregulated, unsafe, and prophylactically inefficient products or Biodefense countermeasures. Oversight was undermined and intellectual property was made unaccessible to government agencies and general the public.
We have established in our previous article that COVID-19 was a military operation in my previous piece, Are COVID-19 ‘Vaccines’ a Military Biodefense Response Gone Terribly Wrong?
As you may have noticed, we did not mention the notorious “Gain of Function” (GoF) research sponsored by the US government’s NIH, but as time goes by we are less inclined to believe GoF can explain everything we’ve experienced since 2020. There must be more to it.
Even according to mainstream science, the so-called ‘novel’ virus dubbed SARS-COV 2 and its alleged “spike protein” (toxin) seem to be presenting characteristics that are not lethal, but that have increased the transmutability and transmission of one or multiple agents designed to mimic viral and bacterial infection symptoms from known pathogens and bacteria. Beyond this, there is still questionable empirical evidence of an isolated sample of the SARSCOV2 virus from one of the initial patients in Wuhan, China in late 2019 and early 2020. Hence, more investigation and public debate are required to prove the epidemiological claims that indeed, a ‘novel coronavirus’ had initiated an unprecedented global plague in 2020.
AI, Synthetic Biology, Biodefense, and Bioweapons
The use of advanced biosynthetic nanotechnologies and Artificial Intelligence (AI) is very likely what the regulators have referred to as a commercial secret that the vaccine manufacturers and the US DoD want to keep some of this under wraps.
In fact, if there is any truth to the Ecohealth Alliance’s DEFUSE PREEMPT proposal to DARPA which offers to vaccinate bats, one could entertain the possibility that persons exposed to a ‘novel coronavirus’ could have, theoretically speaking, been subjected to an aerosol version of a military biosynthetic agent – before receiving an antidote in the form of a COVID-19 ‘vaccine’ as part of a military classification known as “Security Covered Countermeasures”. Certainly, one can see the potential here for an end-to-end problem, reaction, solution, or at the very least a military biodefense ‘solution’ in the age of biowarfare. Regardless of whether this has actually happened, there are already billions of US taxpayers’ dollars being sunk into these very programs as we speak.
Although this may sound far-fetched, the law is clear, the Security Countermeasures are also characterized in the US Code as a “cure” or treatment to a condition that may result in adverse health consequences or death, and may be caused by administering a drug, a biological product, or a device against an agent, or even a biosynthetic agent?
Security Covered Countermeasures characterised (source 1) (source 2):
Of course, many are currently speculating whether or not these exotic bioweapons have anything to do with unlocking the mystery behind the so-called “Origins of Covid (SARS-COV2)”. Whether it is or not may still be an open question, but the system being implemented right before our eyes is very real and largely unregulated.
As you can read for yourself, Security Covered Countermeasures can be a ‘treatment’ or a ‘cure’ against an alleged pandemic – but it can also be characterised as a cure or treatment against any condition caused by either a bioweapon, or ‘Lab Leak’, or even against a pharmaceutical therapy (ie. vaccine) someone may have received in the prevention of (real or perceived) a pandemic or a Chemical, Biological, Radiological, Nuclear (CBRN) threat…. an antidote if you will.
This opens a brand new area of possibilities where a pathogen, a parasite, or a bacteria can be developed from scratch – together with its countermeasures and antidote. If we are right, the scientific discipline that would enable such research et capabilities is known as Synthetic Biology.
If you are still reading this article it is likely you feel, as we do, that something is very wrong with the excentric SARS-COV2 ‘Chinese Lab Leak’ story, and the even more unbelievable fairy tells behind the mRNA COVID-19 vaccines with its “nano-taxis” taking the RNA payload on a guided tour of your body’s cells.
Rest assured that your doubts are valid and that like us, many scientists and journalists around the world have worked countless hours to separate the lies from the truth. Understanding the process and techniques and identifying the hidden technologies and the exotic materials that have been injected into citizens around the world is paramount. The development of these COVID-19 Security Covered Countermeasures and the story of SARS-COV2 are intertwined, and the availability of ‘an antidote’ seems to make sense from a systems analysis point of view, as it is very likely the product of a military research program at DARPA, and BARDA, and with the help of firms like Pfizer and Moderna, therefore, implying military applications developed to protect soldiers against an agent which they might believe to be a Synthetic Bioagent turned into a Synthetic Bioweapon. That’s the military line, but what about civilians? Have such applications now been extended to civilians, courtesy of the US Department of Defense? If so, in what form have they been deployed? Can the experimental mRNA Covid-19 ‘vaccine’ be classified as a bioweapon? That is a fundamental question that must be answered now.
To understand SARS-COV 2 and its countermeasures one must become familiarised with the fast-growing world of synthetic biology and the opportunity it offers to develop Biosynthetic Weapons. Synthetic biology is defined by the US National Academy of Sciences as “concepts, approaches, and tools which enable the modification or creation of biological organisms”, however, the central question here is whether a capability enabled by synthetic biology can be used in such a way as to cause harm — that is, whether such capability can be used as what could be called a “Biosynthetic Weapon” or known in the field as a “Synthetic Biology Enabled Weapon”, to use their terms.
In the below-featured report entitled, “Biodefense in the Age of Synthetic Biology” published in 2018 by the National Academies of Sciences, Engineering, and Medicine, you will be able to discover how biosynthetic biology opens the routes for attempts to re-creating known pathogens, making existing pathogens more dangerous, or creating new pathogens. This report is an eye-opener to better understand the techniques behind the creation of a “Synthetic Biology Enabled Weapon”.
Although it appears that James O’Keefe and his team from Project Veritas may have been set up with the Jordon Walker-Pfizer story, it was hard to avoid the term “Directed Evolution” in this report – as it was used no less than 26 times.
[Go to source to read the 79 page report]
For now, let’s move on and see if we can establish whether or not the EU COVID-19 response was similar to the one observed in the US, a military-like operation responding to a national security CBRN threat rather than a response to a manufactured public health emergency which had had for effect to weaken regulatory power and oversight to keep everyone in the dark.
What was the EU response to COVID-19?
February 4th, 2021, European Union industry commissioner Thierry Breton was put in charge of a new vaccine production task force. It was the European Commission (EC) President Ursula von der Leyen who appointed Breton to lead a Taskforce in view of increasing the industrial production of COVID-19 vaccines.
Breton was interviewed 2 months later on the progress made and he rapidly confirmed that Vaccine developers ( Pfizer, Moderna, etc…) had already enlisted 53 manufacturing sites in Europe and that this number was increasing. Further in the interview, he will confirm what we already knew, which is that these manufacturing sites were in fact Fill & Finish production sites and had nothing to do with the development and the full-scale manufacturing of the so-called Covid-19 vaccines. Further in the interview, he went on to say:
“I have no reason to doubt the potential effectiveness, safety, and quality of vaccines developed outside of the EU, this is a matter for the European Medicines Agency to assess”. (source)
This confirms that the countermeasures and the bio-nano technologies which were developed for these mRNA vaccines were coming from outside of the EU and therefore only Filled & Finished in Europe, a detail Thierry Breton will also touch upon during this interview unfortunately omitting to tell the public that these so-called mRNA vaccines were actually “security covered countermeasures” developed by the US Department of Defense, a detail that would have certainly raised many eyebrows and compromise greatly the vaccination uptake in the European Union.
Therefore we must question the EMA process and their regulatory role for these products which we now know were developed in the US and Canada, and perhaps try to bring answers to the following questions:
1) In which form these products and their technologies developed in the US were brought to the EU?
2) Who was in charge of delivering this experimental product to the EU?
3) How EMA was able to establish the safety and efficacy of this product?
4) Was EMA invited to the US and Canada and authorised to test the final products and control cGMP and CMC before spending billions on an experimental mRNA countermeasure based on Pfizer/BioNTech fraudulent clinical trials
5) Why were CMAs granted despite alarming safety and efficacy data?
6) What was the process to ensure current Good Manufacturing Practices (cGMP) and chemistry, manufacturing, and controls (CMC) which are crucial activities when developing new pharmaceutical products?
What about the European Regulator, the European Medicines Agency – EMA?
The European Medicines Agency (EMA) started a rolling review of preliminary data from Pfizer trials on Oct 6th, 2020 in an effort to speed up the approval process that usually takes at least seven months. The EU had ordered 200 million doses of the Pfizer-BioNTech shot, enough to vaccinate 100 million people, paying 15.50 euros ($18.90) per dose.
The review of Pfizer’s data was undertaken by the EMA’s Committee for Medicinal Products for Human Use (CHMP) the European Medicines Agency’s committee responsible for elaborating the agency’s opinions on all issues regarding medicinal products for human use, CHMP was chaired by Dr. Harald Enzmann. A list of the CHMP members appointed by their respective EU states can be found here.
The EMA eventually published a document to inform the European citizens of the basis for the EU approval for new vaccines, the common denominator being efficacy and safety (around 95%). The said document was put together and presented by Dr. Harald Enzmann on behalf of EMA.
Enzmann was elected on October 15th,2018 for an initial 3 years mission replacing Dr. Tomas Salmonson, a senior scientific advisor at the Swedish Medicinal Products Agency (MPA). Dr. Enzmann is a medical doctor. Previously Enzmann had worked for the Federal Institute for Drugs and Medical Devices (BfArM) in Germany, where he was Head of European and International Affairs. Dr. Enzmann was a member of the CHMP since 2005 and its vice-chair since October 2016.
In Dec 2020, Harald Enzmann declared in a CHMP briefing “The European Medicines Agency (EMA) does not have enough data from the companies’ clinical trials on the potential risks to pregnant women” (source). The reality was that they were simply not enough pregnant women in the Pzifer-BioNTech vaccine clinical trial data to establish safety.
In this EMA report entitled “Basis for the EU approval of new vaccines” put together by CHMP Chair Dr. Enzmann, after two vaccines had already been granted Conditional Marketing Approval (CMA), it is stated:
“It is not yet known to which extend vaccinated people may still be able to carry and spread the so-called virus” when the EMA in the same report tells us: “The vaccines offer a high level of protection around 95%”.
The European mainstream media and their TV studio scientists were advertently misleading the public by promoting the EMA lies, pretending that these mRNA vaccines were safe and efficacious at 95% when in fact no immunity studies were ever conducted by Pfizer prior to EMA granting them a CMA. In our opinion, this is highly irresponsible not to say criminal.
This is, again in our opinion, a piece of undeniable evidence that political pressure was applied to those responsible to approve these vaccines. compromising the safety of EU citizens whilst justifying the liberticide vaccine passports which split the European society in half and accelerated vaccination. People were asked to do their civic duty and protect their families and others based on no evidence whatsoever as admitted by Janine small from Pfizer.
So many caregivers, firemen, and many workers from all walks of life have lost their jobs and livelihood because they dared not trust what the EMA was saying at the time and they were right not to trust them.
Here is the link to the European Commission and EMA press conference approving the use of the Pfizer/BioNTech COVID-19 vaccine in the EU.
Enzmann also said he would urge caution in using the vaccine on people with a known history of anaphylaxis reaction (severe and life-threatening allergic reactions) after several cases of allergic reactions to the vaccine in the United States and the United Kingdom were observed.
Enzmann knew no immunogenicity studies were conducted by Pfizer and it is fair to assume that he knew the spike protein was triggering devastating inflammatory reactions and harmful side effects to anyone subjected to these procedures. He, without doubt, knew the data was too insufficient for them to go to market and the risk v benefits ratio was clearly not in favor of pregnant women yet Pfizer was given the green light. This is reckless at best but that doesn’t explain why would these scientists would want to push out a dangerous medical countermeasure.
“Enzmann defended the independence of the EMA in the approval process despite suggestions in some quarters that there may have been political pressure to speed the process for the EU after Britain had already begun vaccinations,” said a Reuters report. One would be extremely naive to also believe that no political and economical pressure from the US applied.
On December 21st, 2020 the EMA granted Pfizer a Conditional Marketing Authorisation (CMA) which is an approval of a medicine that addresses unmet medical needs of patients on the basis of less comprehensive data than normally required (source). It can be converted into a standard marketing authorisation after further data checks. This would be valid for five years but can be renewed for unlimited validity.
January 6th, 2021, The European Medicines Agency (EMA) granted Moderna a Conditional Marketing Approval (CMA) on the strength that their Spikevax vaccines demonstrated a 94.1% efficacy in the trial but also on the fact that the trial had shown 90.9% efficacy in participants at risk of severe COVID-19, including those with chronic lung disease, heart disease, obesity, liver disease, diabetes or HIV infection.
On March 11th, 2021 the European Medicines Agency (EMA) issued an Assessment report for the COVID-19 Spikevax Vaccine of Moderna following the review of their data by the Committee for Medicinal Products for Human Use (CHMP) the European Medicines Agency’s committee responsible for elaborating the agency’s opinions on all issues regarding medicinal products for human use. This is when some ‘Specific Obligation’ were required from the manufacturers as critical questions were starting to pour in;
• mRNA integrity (SO1)
• characterisation of the active substance, the finished product, and their manufacturing processes
• Consistency of the active substance and finished product manufacturing process
• Stability of the active substance and finished product and review of the active substance and finished product specifications to confirm the quality
We could go on for hours going through all the documents and data and find some of the most disturbing signals revealing an off-balanced risk v benefit ratio that should have halted the deployment of these Security Covered Countermeasures. We certainly have strong ammunitions to destroy the regulators’ and manufacturers’ claims about the safety and efficacy of these countermeasures but what we are truly looking for, is irrefutable evidence a crime was committed. Thanks to some of the best investigative journalists in the place some of this evidence have already been gathered and widely shared. All we need at this stage is brave lawyers going after these alleged criminals and of course your support to share the following data.
Pfizer and BionTech falsify key data
In June 2022, investigative journalist and broadcaster Sonia Elijah author of an investigative report published on Trial Site News broke down the leaked European Medicine Agency (EMA) emails and other Pfizer-related confidential reports, which exposed concerning facts in the run-up to the authorization of the Pfizer-BioNTech COVID-19 vaccine granted by the European Medicines Agency (EMA).
The report reveals how politically motivated the race to approve these mRNA so-called vaccines was. Even more, distributing the report reveals that by late November 2020, regulators including, US FDA, European Medicines Agency, Health Canada, and the UK’s MHRA, were all aware of the significant loss of RNA integrity of the commercial batches (~55% mRNA integrity) of the Pfizer-BioNTech vaccine compared to the clinical ones (~78% mRNA integrity). This was classified by EMA as a “major objection” along with observed visible particles, which were classified as “impurities.”
A leaked 26 November PowerPoint presentation of a meeting between Pfizer-BioNTech and the EMA revealed how this major objection was shockingly ‘resolved’- the RNA integrity specification was simply lowered to 50%, therefore half of all mRNA molecules in the commercial batches were allowed to be truncated (not intact).
“The potential implications of the RNA integrity loss in terms of safety and efficacy were unknown”.
Feb. 4, 2023, Sonia published a new report (Part 1) this time pointing at startling evidence suggesting BioNTech and Pfizer falsified Key Data. The #Blotgate scandal as it is known on social media was never abandoned by the scientific community. The fact there could be actual evidence to prove that Pfizer and BioNTech engaged in fraud by fabricating critical data would have major ramifications and they knew it hence why they kept on pushing.
Links to Sonia Elijah author of the Trial Site News following reports:
Feb. 4, 2023: Startling Evidence Suggests BioNTech and Pfizer Falsified Key Data: Part 1
Earlier this month, Clayton Morris from REDACTED interviewed Investigative journalist Sonia Elijah on the Pfizer & BioNTech cover-up (source)
.
Introducing Part 2
The second part of this series will take you on a journey across Europe where we will able to establish who were the military actors of the EU COVID-19 response whilst understanding who was privy to the fact that these COVID-19 countermeasures were developed by the US Department of Defense with the help of Biodefense / Biowarfare trusted partners such as Moderna and Pfizer and CIA backed mRNA manufacturer “Resilience” “Emergence” amongst many others.
Operation Warp Speed was indeed a military Operation and therefore it totally makes sense for a high-level join EU/NATO Military operation to have served as an interface between US DOD and all EU member states (MS). What we know so far is that US DOD wanted to secure and guarantee the safe delivery of their experimental products whilst hiding their true nature before they could arrive at their final destination, in European citizens’ arms.
In Part 2 we will unpack a different aspect of our European investigation starting with identifying organisations and institutions specialised in military medical services whose task was to strengthen and facilitate civilian efforts in dealing with medical crises or emergencies such as the coronavirus plandemic. With the outbreak of the so-called COVID-19, we know that fighting the plandemic became one of the main priorities for these organisations and that it was executed through civil-military cooperation. The only trusted pan-European actor with logistics expertise and military discipline that has strong ties with the European Union is the North Atlantic Treaty Organization, NATO. In our Part 2, you will also find out how NATO and the EU coordinated a pan-European military operation to protect US DOD Security Covered Countermeasures and their secrets at all costs.
Stay tuned for Part 2…
***
READ MORE COVID NEWS AT: 21st Century Wire Covid Files
ALSO, JOIN OUR TELEGRAM CHANNEL
PLEASE HELP SUPPORT OUR INDEPENDENT MEDIA PLATFORM HERE










No comments:
Post a Comment